No water, no life. Never has a statement being so true. Our planet is covered by 70% water, the remaining 30% consists of emerged land. All the water in the subsoil and on the planet’s surface represent the hydrosphere.
Water is present in any living organism in different concentrations. On average, humans are composed of approximately 80% water, although this value is related to other variables such as age, sex and weight.
At the bottom of the post you’ll find an helpful book: “Water” by ‘Wikipedia, The Free Encoclopedia’. Don’t miss it, download it now. You will also find an image for download: “A diagram of the water cycle”. You can complete it, print it, and color it.
Here is a table that relates these variables.
Total body water in % of weight
| Child | Man | Woman | |
|---|---|---|---|
| Thin | 80% | 65% | 55% |
| Normal | 70% | 60% | 50% |
| Obese | 65% | 55% | 45% |
The universe is composed of matter, this has physical characteristics that affect its shape, its color and its density. The three main states (from the physical point of view) of matter are: solid, liquid and gaseous. Water is the only substance which is found in nature in all three states of aggregation.
Normally we see the water in its liquid state in the form of rain, dew or large quantities like oceans, seas, lakes and rivers. In gaseous state it assumes the form of fog and steam which are the main constituents of clouds. Finally in the solid state it is present in the form of ice, snow, hail, frost and clouds.
It seems incredible, but the amount of water we have is always the same and it has been present on our planet for millions of years. This moves continuously in the environment taking on the different forms of which we spoke. This transformation and movement process is called the water cycle.
Let’s see, in short, how it works and I refer you to article – The water cycle on the blog ‘Angolo dei Bimbi’ – (a partner) where the topic is covered in detail.
WATER CYCLE PHASES
– Step 1 – Evaporation: The sun heats up the water surface which evaporates and becames water vapor. The vapor, being lighter, goes into the atmosphere.
– Step 2 – Condensation: Water vapor cools down and condenses, forming clouds.
– Step 3 – The clouds continue to swell and once they encounter cold air, they come together, gain weight, and turn into rain (precipitation phenomenon), falling again on Earth from where it all started with evaporation . If the temperature change is very abrupt, the rain becomes snow or hail.
– Step 4 – The water that falls on earth forms rivers (surface runoff) or ends up in the soil (infiltration). This underground water feeds the groundwater, lakes and rivers. At this point a new cycle starts again.
Returning to the physical states of water, lets try to understand what happens to the molecules when matter changes state.
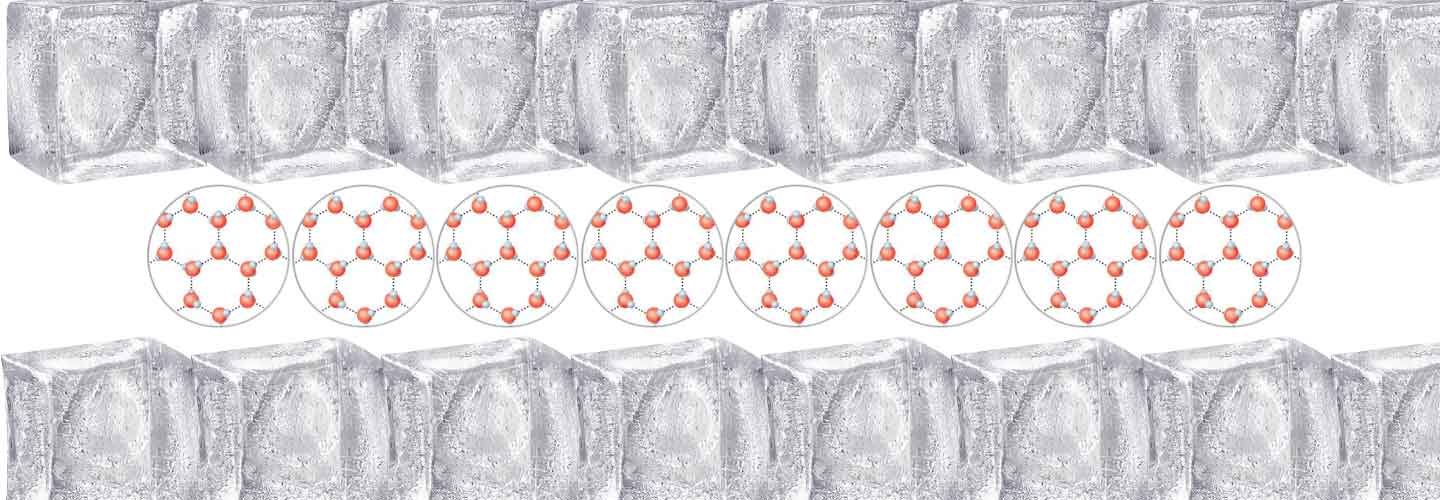
The solids have their own shape and volume. Due to the strong link among molecules, these are arranged according to a certain order . If you add energy, they heat up, some bonds are broken thereby obtaining a liquid.
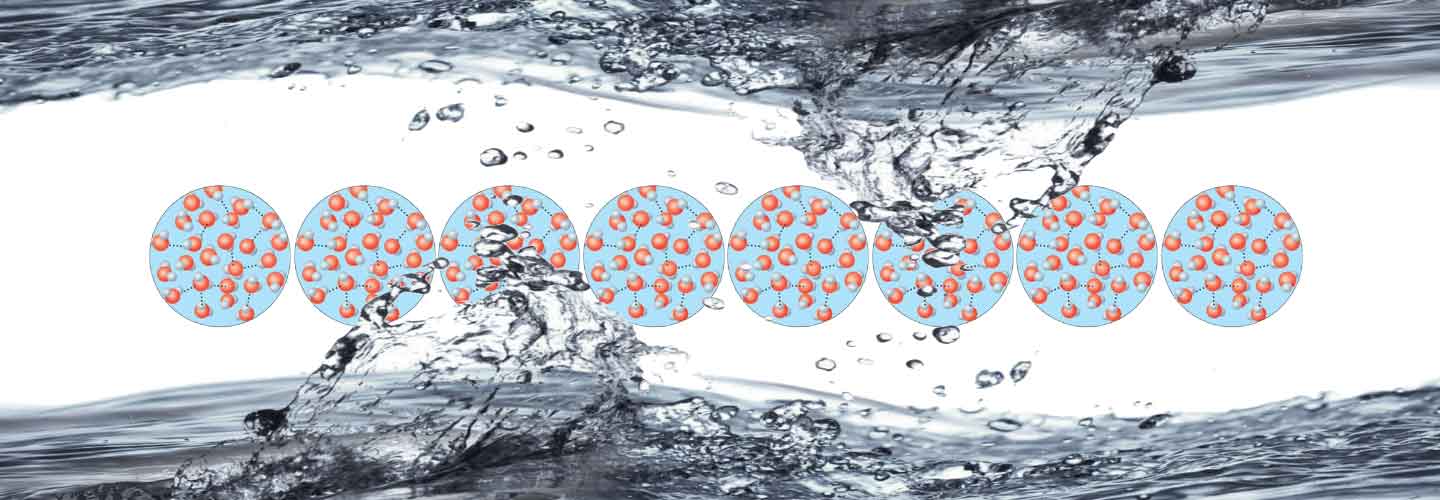
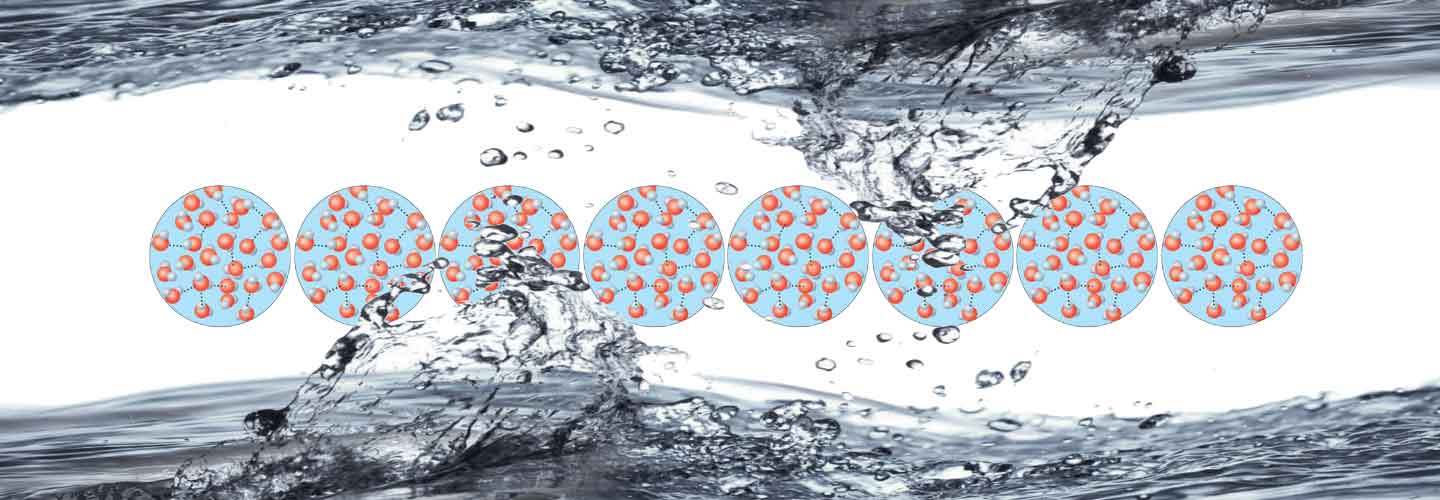
Liquids don’t have form, but possess volume which fills the spaces of the objects whicht contain them. A higher energy than the above mentioned one, breaks all the bonds among molecules turning liquid into gas.
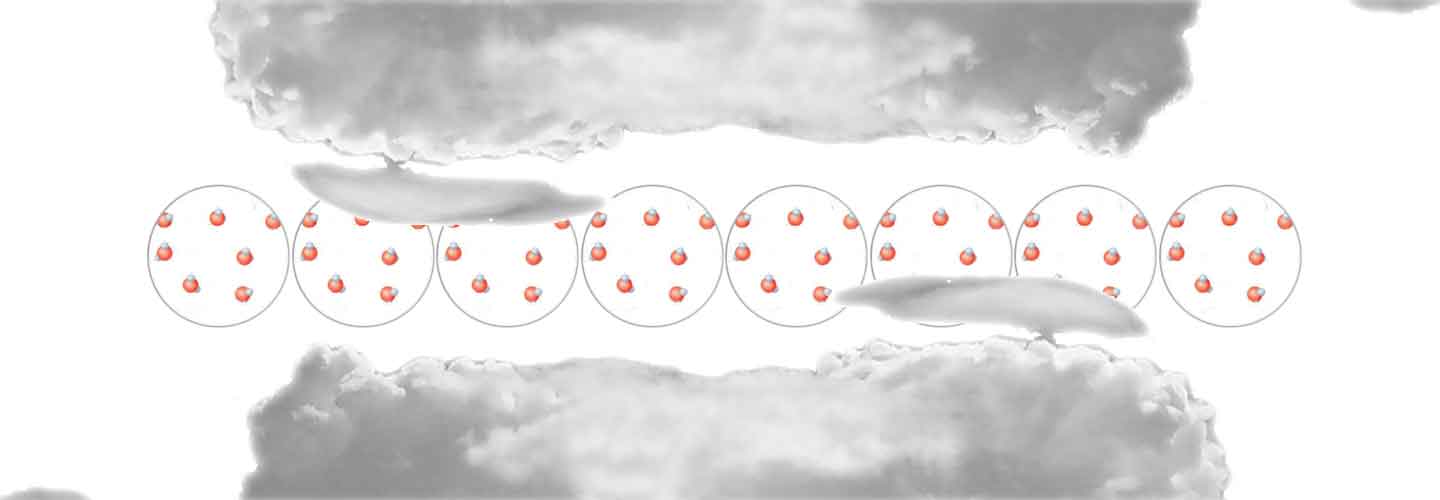
The gas particles are in constant motion making it shapeless and volumeless.
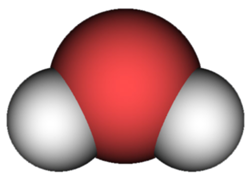
Water assumes a different form, but in reality the molecules are always the same. The different states of matter are thus a transformation of the physical aspect and not of the molecular characteristics. The water in any of its states is always a chemical compound, a union of two different elements: oxygen and hydrogen. Two hydrogen atoms with positive charge are joined to an oxygen atom with two negative charges, forming the molecule H2O (chemical formula of water).
In relation to changes of temperature and pressure (atmospheric pressure is mostly constant), we can identify the change water undergoes.
At 0° C (referred to as melting point, the solid phase and the liquid phase coexist.) water becomes ice, increasing in volume; at 100 ° C (referred to as boiling point) water starts to boil and evaporate.
Ice solidifies very slowly from the surface down; this is vital for the sea inhabitants, which would otherwise remain trapped.
Additional sources for learning:
- Water. (2016, October 9). In Wikipedia, The Free Encyclopedia. Retrieved 20:11, October 9, 2016, from https://en.wikipedia.org/w/index.php?title=Water&oldid=743468788
- During my research I came across a site ‘http://cicloacqua.altervista.org/‘ created as a teaching exercise by a school that addressed the issue extensively.
Video (experiment with english subtitles)
Gallery
(Before / After)
Solid -> Liquid (Fusion)
Liquid -> Gaseous (Evaporation)
Solid -> Gaseous (Sublimation)


Gaseous -> Solid (Frosting)


(Other)
- H2O water molecule
- Water molecules linked together
- Ice
- Water fountain
- Human body composition
- Water cycle
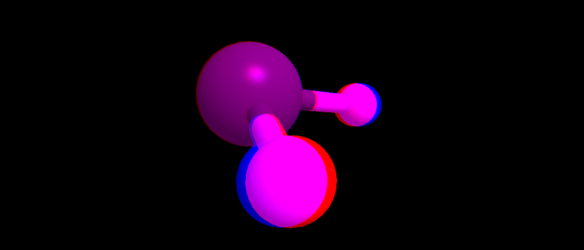
- Anaglyph of the water molecule
- Cross-Eyed 3D model of the water molecule











 (+2 score, 2 raters)
(+2 score, 2 raters)